Blog
How Accelerating eCOA Implementation Benefits Decentralized Clinical Trials
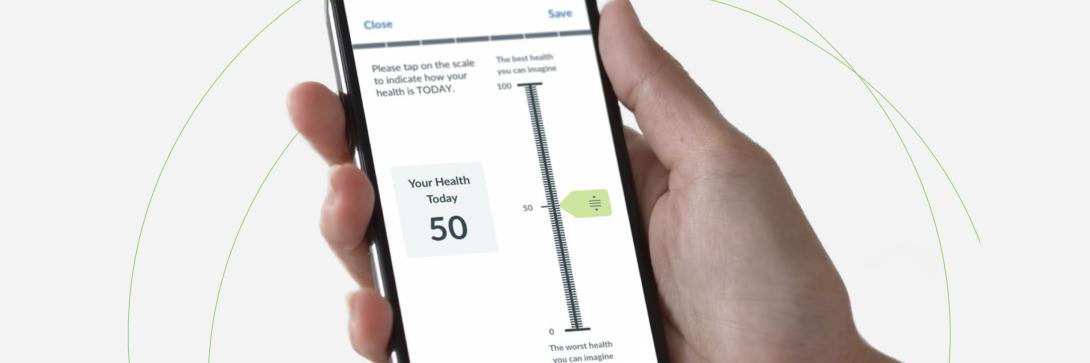
Before COVID-19, the clinical trial space was already increasingly implementing remote data collection. Regulatory agencies across Europe and North America began publishing best practice guides that included recommendations for both paper and virtual formats.
“Virtual trials have been in use for nearly a decade, but only recently did the industry see the scale of benefits it delivered,” said Lisa Charlton of Science 37. “COVID-19 restrictions increased awareness for trial support outside the brick-and-mortar site—proving the model works.”
Evidence for decentralized trials' success
A more remote approach to clinical trials improves speed, retention, and diversity. While decreased time recruiting provides broader access to patients and investigators, broadening the field of participants.
Charlton noted that clients working with Science 37 experience enrollment 21 times faster, a tripling of patient diversity, and nearly 30 percent higher participant retention over the lifespan of a study. This increase results in earlier data collection, expanded data points, and more accurate conclusions for a broader group of potential patients.
“Increased participant recruitment and retention means better outcomes for everyone,” said Lionbridge’s [Dan Herron]. “Language is a key element to unlock universal access.”
Technology implementation must match the pace of decentralized trials
A key benefit of decentralization is the ability to execute faster. Technology capabilities and vendor support enable faster implementation of trials, greater speed to go live, and accelerated timelines.
Faster execution has significant cost benefits. With an accelerated implementation of trial technology, study teams are gaining three to four months of study startup time, potentially equating to $120M and $240M in Opportunity Cost Savings.
Electronic clinical outcome assessments (eCOA) are critical to decentralized trials as they capture the patient voice, but they are a challenge to quickly implement. Multiple stakeholders must adhere to myriad procedures from copyright permissions to IRB/EC submissions and translations.
Standard eCOA processes often require a series of time-consuming steps, including Licensing, Design, Build, Author Approval of Screen Shots, Testing / UAT and Translations, IRB/EC submissions, and Deployment. On average, an eCOA process takes 12-16 weeks to complete without licensing and translations (for US English).
Licensure paths and the importance of timing
The impact of cascading timing and required translations makes licensing a priority for any administrator. A reliable baseline estimate for obtaining licensing is four to six weeks from first contact with the copyright owner to license execution. This timeframe will extend based on scope, the number of translated languages, and administration modality.
The flow of COA licensing can require an overwhelming number of ongoing conversations, which is why using an experienced vendor saves time and resources. Lionbridge maintains an internal eCOA Information Library that tracks conditions of use and copyright information for all of the licensing services we have provided to COAs. “This library is constantly maintained and updated to ensure the information is as accurate and diverse as possible,” said Herron.
Science 37’s library of pre-configured and/or pre-approved assessments aligns with the information provided by licensing services. This pre-configured, pre-approved eCOA library allows study teams to accelerate towards translations, improving timelines.
Language and licensing
Administrators need to know every facet of their assessments when considering electronic measuring and reporting across linguistic barriers. In the “How to Accelerate eCOA Implementation” Insight Brief, Science 37 and Lionbridge share the translation steps and methodology to expedite the eCOA translation process. Click here to access these important steps and learn more about eCOA implementation.